Verily announced a health watch in 2017, with the ability to give insights into medical conditions such as PTSD and Parkinson’s disease. One of the main focus areas for Verily, Alphabet’s health division, is it’s prescription-based Study Watch and its ability to gauge cardiovascular health. Verily received Class II FDA clearance for the watch’s electrocardiogram (ECG) technology.
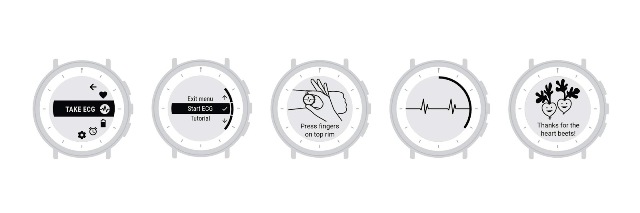 The watch can display and record single-channel ECG rhythms, and along with the research trials, physicians can prescribe the device to people as part of their health care. “Receiving this clearance showcases our commitment to the high standards of the FDA for safety and effectiveness and will help us advance the application of Study Watch in various disease areas and future indications,” Verily said in a recent post.
The watch can display and record single-channel ECG rhythms, and along with the research trials, physicians can prescribe the device to people as part of their health care. “Receiving this clearance showcases our commitment to the high standards of the FDA for safety and effectiveness and will help us advance the application of Study Watch in various disease areas and future indications,” Verily said in a recent post.
 While the Verily Study Watch isn’t a consumer product, it now does have FDA approval, which means that it could appear in some upcoming smartwatches other than Apple’s iWatch, such as the Wear OS line. It’s worth noting that Google just bought smartwatch tech from Fossil for $40 million, verifying its commitment to wearables. With Apple’s now very popular ECG function on their iWatch 4, it’s likely Google wants to offer more health-based options within its smartwatch line-up to compete.
While the Verily Study Watch isn’t a consumer product, it now does have FDA approval, which means that it could appear in some upcoming smartwatches other than Apple’s iWatch, such as the Wear OS line. It’s worth noting that Google just bought smartwatch tech from Fossil for $40 million, verifying its commitment to wearables. With Apple’s now very popular ECG function on their iWatch 4, it’s likely Google wants to offer more health-based options within its smartwatch line-up to compete.





